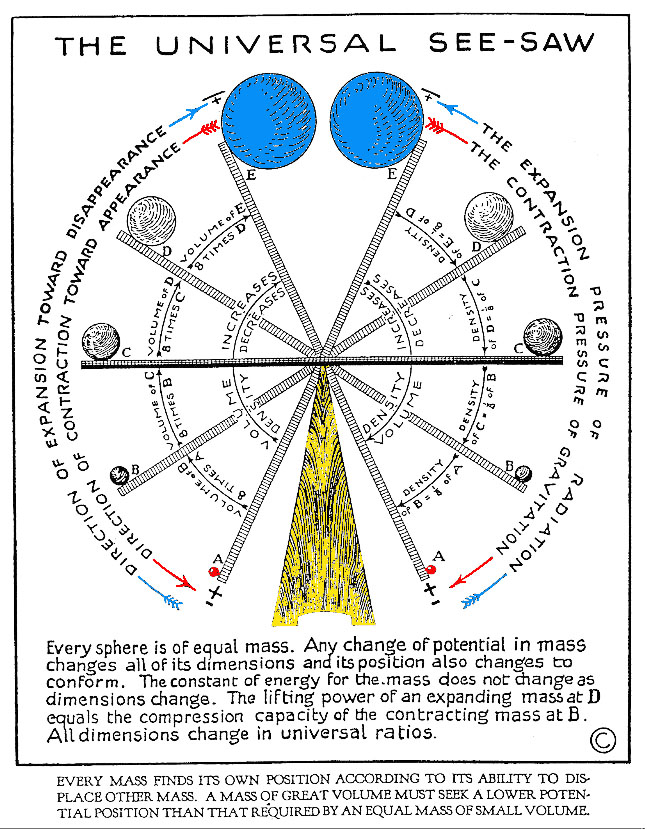
Universal Seesaw
(courtesy University of Science and Philosophy)
(click to enlarge)
Redox reactions, or oxidation-reduction reactions, have a number of similarities to acid-base reactions. Fundamentally,
redox reactions are a family of reactions that are concerned with the transfer of electrons between species. Like acid-base reactions,
redox reactions are a matched set - you don't have an oxidation reaction without a
reduction reaction happening at the same time. Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons. Each reaction by itself is called a "half-reaction", simply because we need two (2) half-reactions to form a whole reaction.
http://www.shodor.org/unchem/advanced/redox/
See Also
cycle of motion
dual character of force
Indig Numbers
Ion
Ionization
Oxidation Number
production of the opposite effect
Reduction
Reduction potential
Valence