| Electron | Electron | |
Sympsionics Symbol | ||
Feb 15, 1826 - George Johnstone Stoney is born. In 1874 he proposes the existence of the electron as a fundamental unit of charge, and coins the word "electron" on 26 March 1891. The electron is the lightest stable subatomic particle known. It carries a negative charge [see syntropy] which is considered the basic charge of electricity. The electron may also be viewed as a unit of attractive force or syntropy.
Keely
"Were our sight able to penetrate the interstitial spaces that exist inside the orbits of the oscillating intermolecules and analyze the conditions in those interstitial spaces, where dwells incalculable latent energy, we would be bewildered with amazement. And assuming our vision, which is limited by persistency, could follow the intermolecules in their rapid oscillations and the intermolecular etheric capsule as it revolves with infinite velocity like a transparent shell about the three component atoms that exist inside it, which in turn revolve in their orbits and oscillate with even a higher frequency than the intermolecules, we would still be only on the border gazing into the remote depths of the interstitial realms that stretch far down into the interatomic, etheric and interetheric subdivisions, and, within the interetheric subdivision at last arrive at the neutral center, the nucleus of everything we know as substance. This neutral center bears about the same relation to the etheric subdivision that the atomic subdivision bears to the crude molecular, in other words, its texture is as much finer than electrons as electrons are finer than coarse molecules." [INTERSTITIAL SPACES]
Electron is therefore a quantity not a thing.
All light particles are either expressing the mother-light principle or the father-light principle. For example, if a particle is on the amplitude of the wave, it would be a true sphere, and as a true sphere it would be neither positive nor negative. It might then appropriately be called a neutron. A particle which is spirally heading inward toward the apex of a vortex in the process of becoming a sphere might appropriately be called a proton, because of its expressing the father-light principle.
?Again, if it is moving spirally outward, it could appropriately be called an electron because it would then be discharging in excess of its charge or expanding in excess of its contraction.
Light rays, for example, leaving the sun, are discharging the sun. They are also discharging themselves because they are expanding into greater volume. They are also lowering their own potential by multiplying their volume. They reverse their polarity when radially converging upon the earth. They are then charging the earth and themselves by contracting into smaller volume and are simultaneously multiplying their own potential by thus contracting. [Walter Russell, The Secret of Light, pages 166-167]
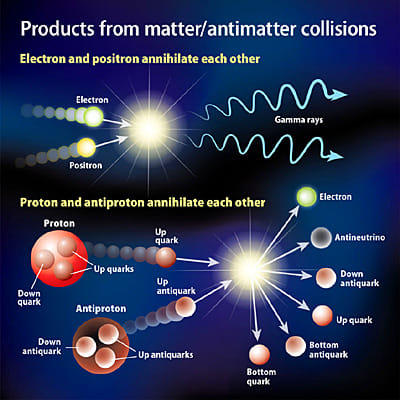
An electron is nearly massless. It has a rest mass of 9.1x10-28 gram, which is only 0.0005 the mass of a proton. The electron reacts only by the electromagnetic, weak, and gravitational forces; it does not respond to the short-range strong nuclear force that acts between quarks and binds protons and neutrons in the atomic nucleus. The electron has an antimatter counterpart called the positron. This antiparticle has precisely the same mass and spin, but it carries a positive charge. If it meets an electron, both are annihilated in a burst of energy. Positrons are rare on the Earth, being produced only in high-energy processes (e.g., by cosmic rays) and live only for brief intervals before annihilation by electrons that abound everywhere.
The electron was the first subatomic particle discovered. It was identified in 1897 by the British physicist J. J. Thomson during investigations of cathode rays. His discovery of electrons, which he initially called corpuscles, played a pivotal role in revolutionizing knowledge of atomic structure.
Under ordinary conditions, electrons are bound to the positively charged nuclei of atoms by the attraction between opposite electric charges. In a neutral atom the number of electrons is identical to the number of positive charges on the nucleus. Any atom, however, may have more or fewer electrons than positive charges and thus be negatively or positively charged as a whole; these charged atoms are known as ions. Not all electrons are associated with atoms. Some occur in a free state with ions in the form of matter known as plasma. (source unknown)
Most of the matter we see around us is made from protons and neutrons, which are each composed of 3 quarks. There are six quarks, or quark flavours, but physicists usually talk about them in terms of three pairs: up/down, charm/strange, and top/bottom. Top and bottom types are the most elementary of them all, and are the ones that make up protons and neutrons. Quarks have the unusual characteristic of having a fractional electric charge, unlike the proton and electron, which have integer charges of +1 and -1 respectively. The up, charm and top quarks have a charge of +2/3, whilst the down, strange and bottom have a charge of -1/3. Although individual quarks have fractional electrical charges, they normally combine into 'hadrons' such that these hadrons have a net integer electric charge. Protons and neutrons are good examples of quark grouping or hadrons. Researchers at the Weizmann Institute of Science have provided the first unambiguous evidence that electrons can behave in an intriguing way that seems to defy the idea of the electron being an indivisible charged elementary unit. [See 13.06 - Triple Currents of Electricity]
The electron can be said to be the quantum of the Dirac field through second quantization of the Dirac equation, which also leads to the prediction of the existence of the positron as another quantum of this field with the same mass but with a charge opposite to that of the electron.
One prominent representative of the lepton species, which comes in three families, each with an electrically charged lepton and a neutral neutrino, is the electron. It is known to play the all-important role in chemical and electrical phenomena. According to present-day knowledge, the leptons are elementary, i.e., are indivisible and have no substructure.
The proton (and neutron), i.e., nucleur matter, on the other hand, has been shown to be composed of more elementary units, the quarks. Supposedly six different kinds of quarks exist, again grouped in three families, in complete symmetry to the leptons. Five of these quarks have been found, and recent experimental evidence exists for the sixth quark. Only the two members of the first family are needed for "ordinary" nuclear matter, the "u" and the "d" quark. However, the quarks seem to always be bound together to form the nuclear particles, called hadrons, and never have been observed as free particles: Three such quarks make up the proton, two u quarks and one d quark, while the neutron consists of one u quark and two d quarks. [See 16.29 - Triple Currents of Electricity]
Hadrons containing the heavier quarks, s, c, and b have also been observed. These particles, however, have very short lifetimes and are being produced under "natural" condition(s) mostly in reactions of cosmic rays with nuclei in the terrestrial atmosphere. [McGraw-Hill Concise Encyclopedia of Science & Technology]
Gustave Le Bon
"It is thus that Kaufmann deduces from his observations that the electron, of which certain radio-active emissions are composed, "is nothing but an electric charge distributed over a volume or a surface of very small dimensions." [Gustave Le Bon, The Evolution of Matter, page 191]
"Electron is a name of a uniform field that can be concentrated in a point by being sufficiently impacted by an imposed outer force." [anonymous]
Christ Returns - Speaks His Truth
"Fifthly, I have come expressly to help science bridge the gulf between UNIVERSAL CONSCIOUSNESS and the appearance of electrical particles. Without this bridge between the Unseen Spiritual Dimension and the Seen world of 'matter', science will remain rooted in old ideas and concepts instead of moving forward into new realms of spiritual/scientific research for the betterment of mankind." [Christ Returns - Speaks His Truth, Letter 5]
Structure:???
Lepton Neutral neutrino Electron
Characteristics:
01 - is a lepton (light particles). 02 - Plays important role in chemical and electromagnetic interactions. 03 - least massive electrically charged particle, therefore absolutely stable. 04 - most common lepton with charge -1. 05 - held to be elementary, i.e., are indivisible and have no substructure. 06 - is a Fermion (mutually repulsive). 07 - obeys Fermi-Dirac Statistics. 08 - is subject to the Pauli Exclusion Principle. 09 - half-integer values of spin. 10 - wavefunction must be antisymmetric with respect to the exchange of identical particles. 11 - Electrons, as fermions, act on each other by exchanging bosons. 12 - As a fermion, there can be only one electron for each state in an atom. 13 - does not participate in strong interactions. 14 - does participate in weak interactions. 15 - Fundamental, as far as we know, an electron cannot be broken down into smaller particles.
Antiparticle: Positron (electron number: -1) ? Electron Number: +1 ?
Quarks, leptons and baryons are all fermions.
Electron: One prominent representative of the lepton species, which comes in three families, each with an electrically charged lepton and a neutral neutrino, is the electron. It is known to play the all-important role in chemical and electrical phenomena.
The least massive electrically charged particle, therefore absolutely stable. It is the most common lepton with charge -1. An electron is one of the fundamental particles in nature. Fundamental means that, as far as we know, an electron cannot be broken down into smaller particles. (This concept is one of the things SLAC physicists always challenge by looking for other particles.) Electrons are responsible for many of the phenomena that we observe in everyday life. Mutual repulsion between electrons in the atoms of the floor and those within your shoes keeps you from sinking and disappearing into the floor!!! Electrons carry electrical current and successful manipulation of electrons allows electronic devices, such as the one you are using, to function.
Fermion - any particle that obeys Fermi-Dirac statistics and is subject to the Pauli exclusion principle
Fermion: A particle, such as the electron, proton, or neutron, which obeys the rule that the wave function of several identical particles changes sign when the coordinates of any part are interchanged; it therefore obeys the Pauli exclusion principle.
Fermions have half-integer values of spin [see BOSON which have integer value spin]
An odd half-integer spin particle. Fermions act on each other by exchanging bosons. Examples include leptons (such as the electron), neutrons, protons and quarks. They are indistinguishable, have antisymmetric wave functions, and obey Fermi-Dirac statistics. Fermions obey Fermi-Dirac statistics.
Fermions are particles which have half-integer spin and therefore are constrained by the Pauli exclusion principle. Particles with integer spin are called bosons. Fermions incude electrons, protons, neutrons. The wavefunction which describes a collection of fermions must be antisymmetric with respect to the exchange of identical particles, while the wavefunction for a collection of bosons is symmetric.
The fact that electrons are fermions is foundational to the buildup of the periodic table of the elements since there can be only one electron for each state in an atom (only one electron for each possible set of quantum numbers). The fermion nature of electrons also governs the behavior of electrons in a metal where at low temperatures all the low energy states are filled up to a level called the Fermi energy. This filling of states is described by Fermi-Dirac statistics.
Electron TIME OF ROTATION (Orbit)
"The frequency of "rotation" of an electron around the nucleus is of the order of 10-16 second, the typical lifetime 10-9 second. Therefore an excited electron rotates 10,000,000 times before it falls down to the ground state." — Ilya Prigogine, From Being to Becoming: Time and Complexity in the Physical Sciences
Structure:
Considered Fundamental
Characteristics:
01 - does not participate in strong interactions.
02 - light as opposed to heavy
03 - NOT made of quarks
04 - participates in weak interactions.
05 - has baryon number of 0
06 - half-spin particle
07 - is fermion
08 - fermion having a mass smaller than the proton mass
09 - interact with electromagnetic and gravitational fields
10 - considered fundamental
A fundamental matter particle that does not participate in strong interactions. The charge leptons are the electron (e), the muon (), the tau () and their antiparticles. Neutral leptons are called neutrinos ( ).
A lepton (Greek for "light", as opposed to hadrons which are "heavy") is a subatomic particle that is not made of quarks. See 14.25 - Dominant is Light of Electrical Spark
An elementary particle that participates in weak interactions; has a baryon number of 0.
Lepton : A collective term for those spin 1/2 particles (Fermions) which do not undergo strong interactions. The word Lepton was coined from Greek root to indicate that these are light particles. The known leptons (e;, m;, ne, nm) are all lighter than the mesons and baryons. [FermiLab] See 14.25 - Dominant is Light of Electrical Spark
A fermion having a mass smaller than the proton mass; leptons interact with electromagnetic and gravitational fields, but beyond this they interact only through weak interactions.
One prominent representative of the lepton species, which comes in three families, each with an electrically charged lepton and a neutral neutrino, is the electron. It is known to play the all-important role in chemical and electrical phenomenoa. According to present-day knowledge, the leptons are elementary, i.,. are indivisible and have no substructure.
The proton (and neutron), i.e., nuclear matter, on the other hand, has been shown to be composed of more elementary units, the quarks. Supposedly six different kinds of quarks exist, again grouped in three families, in complete symmetry to the leptons. Five of these quarks have been found, and recent experimental evidence exists for the sixth quark. Only the two members of the first family are needed for "ordinary" nuclear matter, the "u" and the "d" quark. However, the quarks seem to always be bound together to form the nuclear particles, called hadrons, and never have been observed as free particles: Three such quarks make up the proton, two u quarks and one d quark, while the neutron consists of one u quark and two d quarks.
Hadrons containing the heavier quarks, s, c, and b have also been observed. These particles, however, have very short lifetimes and are being produced under "natural" conditions mostly in reactions of cosmic rays with nuclei in the terrestrial atmosphere.
A particle (like the electron, muon, and neutrino) that participates in the weak, but not the strong, interactions.
A class of fermion whose members participate in weak, electromagnetic, and gravitational interactions. Every lepton has a corresponding antilepton. All leptons have lepton number 1, while all antileptons have lepton number -1.
Leptons
Leptons and quarks are the basic building blocks of matter, i.e., they are seen as the "elementary particles". There are six leptons in the present structure, the electron, muon, and tau particles and their associated neutrinos. The different varieties of the elementary particles are commonly called "flavors", and the neutrinos here are considered to have distinctly different flavor.
Important principles for all particle interactions are the conservation of lepton number and the the conservation of baryon number.
Now that we have experimental evidence for six leptons, a relevant question is "Are there more?". The present standard model assumes that there are no more than three generations. One of the pieces of experimental evidence for that is the measured hydrogen/helium abundance ratio in the universe. When the process of nucleosynthesis from the big bang is modeled, the number of types of neutrinos affects the abundance of helium. The observed abundance agrees with three types of neutrinos.
"Hot electrons are typically generated through shining a certain frequency of light on a carefully engineered nanostructure made of metals such as gold or silver, exciting so-called "surface plasmons." These plasmons are believed to eventually lose some of their energy to electrons, making them hot." https://phys.org/news/2020-06-discovery-hot-electrons-efficient-energy.html
See Also
Bearden on Tesla and EM Source Charge
Bohr Magneton
Charge
charged body
doubly charged mass
Electricity
Electron Motions
Electron Stahls
elementary charge
Etheric Elements
Interatom
Interatomic
Intra-atomic energy
Mind Force is a pre-existing Natural Force
Neutron
Particles and Corpuscles
Proton
Quantum Chronology
Quark
Reduction potential
space charge
Sympsionics
Table of Quantum Particles
12.35 - End of The Electron Theory
16.29 - Triple Currents of Electricity