| Neutron | Neutron | |
Sympsionics Symbol | ||
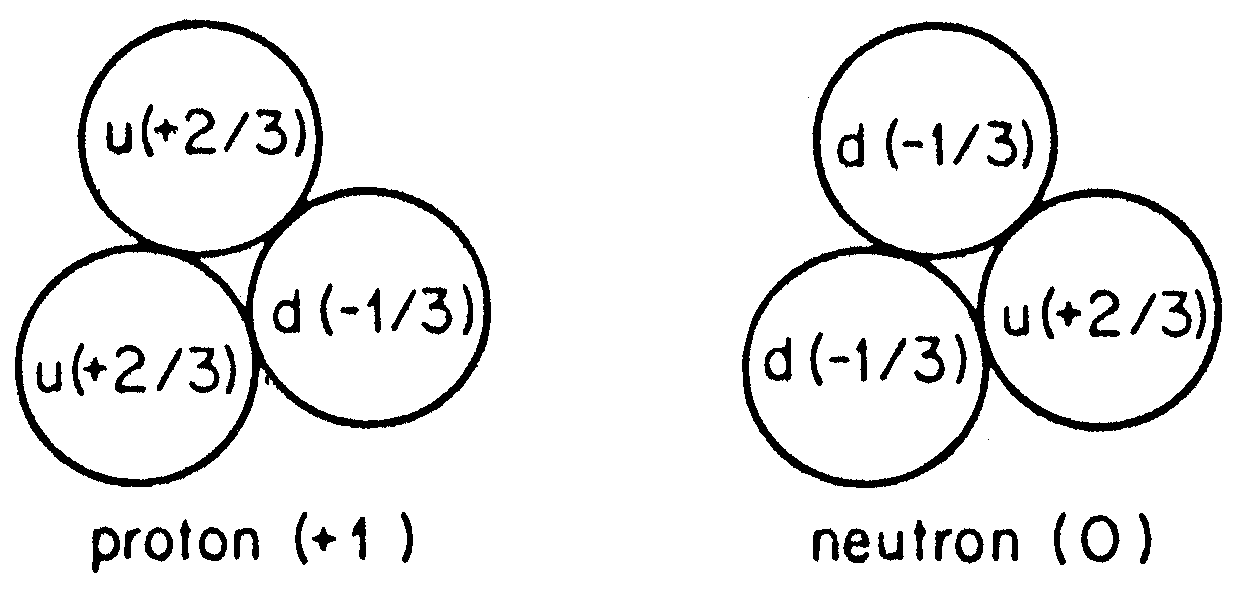
Richard Feynman's Structure of the Proton and Neutron from his book, "QED"
Russell
All light particles are either expressing the mother-light principle or the father-light principle. For example, if a particle is on the amplitude of the wave, it would be a true sphere, and as a true sphere it would be neither positive nor negative. It might then appropriately be called a neutron. A particle which is spirally heading inward toward the apex of a vortex in the process of becoming a sphere might appropriately be called a proton, because of its expressing the father-light principle.
?Again, if it is moving spirally outward, it could appropriately be called an electron because it would then be discharging in excess of its charge or expanding in excess of its contraction.
Light rays, for example, leaving the sun, are discharging the sun. They are also discharging themselves because they are expanding into greater volume. They are also lowering their own potential by multiplying their volume. They reverse their polarity when radially converging upon the earth. They are then charging the earth and themselves by contracting into smaller volume and are simultaneously multiplying their own potential by thus contracting. [Walter Russell, The Secret of Light, pages 166-167]
[PHYS] An elementary particle which has approximately the same mass as the proton but lacks electric charge and is a constituent of all nuclei having mass number greater than 1.
One prominent representative of the lepton species, which comes in three families, each with an electrically charged lepton and a neutral neutrino, is the electron. It is known to play the all-important role in chemical and electrical phenomenoa. According to present-day knowledge, the leptons are elementary, i.e., are indivisible and have no substructure.
The proton (and neutron), i.e., nucleur matter, on the other hand, has been shown to be composed of more elementary units, the quarks. Supposedly six different kinds of quarks exist, again grouped in three families, in complete symmetry to the leptons. Five of these quarks have been found, and recent experimental evidence exists for the sixth quark. Only the two members of the first family are needed for "ordinary" nuclear matter, the "u" and the "d" quark. However, the quarks seem to always be bound together to form the nuclear particles, called hadrons, and never have been observed as free particles: Three such quarks make up the proton, two u quarks and one d quark, while the neutron consists of one u quark and two d quarks.
Hadrons containing the heavier quarks, s, c, and b have also been observed. These particles, however, have very short lifetimes and are being produced under "natural" condition[s] mostly in reactions of cosmic rays with nuclei in the terrestrial atmosphere. [McGraw-Hill Dictionary of Science & Technical Terms 3rd ed.]
A particle of approximately the same mass as the proton, but uncharged. [Trefil, James S.; From Atoms to Quarks - An Introduction to the Strange World of Particle Physics; Charles Scribner's Sons, New York, 1980]
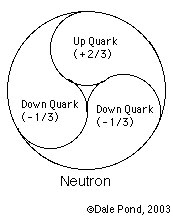
Neutron
Hadrons are nuclear particles. Neutron is a Hadron.
Structure:
Down Quark 2/3
Down Quark 2/3
Up Quark -1/3
Characteristics:
01 - neutron consists of one up quark and two down quarks.
02 - A baryon with electric charge zero.
03 - particle which has approximately the same mass as the proton.
04 - lacks electric charge
05 - constituent of all nuclei having mass number greater than 1.
06 - composed of more elementary units, the quarks.
07 - is a nuclear particle, called hadrons.
08 - is a fermion.
09 - emits and absorbs pions with/from protons.
10 - affected only by Strong Force.
11 - not affected by magnetic or electric charge.
Neutron From Wikipedia, the free encyclopedia.
In physics, the neutron is a subatomic particle with no net electric charge and a mass of 940 MeV (very slightly more than a proton). Its spin is 1/2. The nucleus of most atoms (all except the most common isotope of Hydrogen, which consists of a single proton only) consists of protons and neutrons. Outside the nucleus, neutrons are unstable and have a half-life of about 15 minutes, decaying by emitting an electron and antineutrino to become a proton. The same decay method (beta decay) occurs in some nuclei. Particles inside the nucleus are typically resonances between neutrons and protons, which transform into one another by the emission and absorption of pions. A neutron is classified as a baryon, and consists of two down quarks and one up quark.
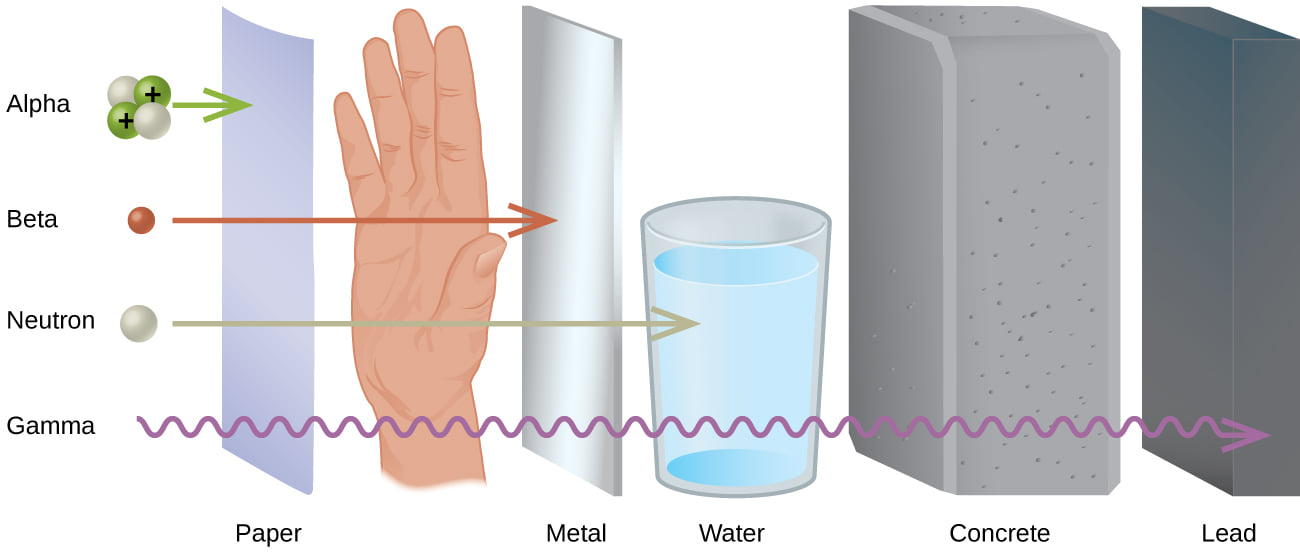
The characteristic of neutrons which most differentiates them from other common subatomic particles is the fact that they are (9depolar|uncharged)). This property of neutrons delayed their discovery, makes them very penetrating, makes it impossible to observe them directly, and makes them very important as agents in nuclear change.
Although atoms in their normal state are also uncharged, they are ten thousand times larger than a neutron and consist of a complex system of negatively charged electrons widely spaced around a positively charged nucleus. Charged particles (such as protons, electrons, or alpha particles) and electromagnetic radiations (such as gamma rays) lose energy in passing through matter. They exert electric forces which ionize atoms of the material through which they pass. The energy taken up in ionization equals the energy lost by the charged particle, which slows down, or by the gamma ray, which is absorbed. The neutron, however, is unaffected by such forces; it is affected only by the very short-range strong nuclear force which comes into play when the neutron comes very close indeed to an atomic nucleus. Consequently a free neutron goes on its way unchecked until it makes a "head-on" collision with an atomic nucleus. Since nuclei have a very small cross section, such collisions occur but rarely and the neutron travels a long way before colliding.
nuclear weapons and nuclear reactors effects are of great practical importance in
apply as they do in the elastic collision of billiard balls. If the nucleus that is struck is heavy, it acquires relatively little speed, but if it is a proton, which is approximately equal in mass to the neutron, it is projected forward with a large fraction of the original speed of the neutron, which is itself correspondingly slowed. Secondary projectiles resulting from these collisions may be detected, for they are charged and produce ionization.
The uncharged nature of the neutron makes it not only difficult to detect but difficult to control. Charged particles can be accelerated, decelerated, or deflected by electric or magnetic fields which have no effect on neutrons. Furthermore, free neutrons can be obtained only from nuclear disintegrations; there is no natural supply. The only means we have of controlling free neutrons is to put nuclei in their way so that they will be slowed and deflected or absorbed by collisions. These effects are of great practical importance in nuclear reactors and nuclear weapons.
The neutron is a subatomic particle, symbol n or n0, with no net electric charge and a mass slightly larger than that of a proton. Protons and neutrons, each with mass approximately one atomic mass unit, constitute the nucleus of an atom, and they are collectively referred to as nucleons.5 Their properties and interactions are described by nuclear physics.
The nucleus consists of Z protons, where Z is called the atomic number, and N neutrons, where N is the neutron number. The atomic number defines the chemical properties of the atom, and the neutron number determines the isotope or nuclide. The terms isotope and nuclide are often used synonymously, but they refer to chemical and nuclear properties, respectively. The atomic mass number, symbol A, equals Z+N. For example, carbon has atomic number 6, and its abundant carbon-12 isotope has 6 neutrons, whereas its rare carbon-13 isotope has 7 neutrons. Some elements occur in nature with only one stable isotope, such as fluorine. Other elements occur as many stable isotopes, such as tin with ten stable isotopes. Even though it is not a chemical element, the neutron is included in the table of nuclides.
Within the nucleus, protons and neutrons are bound together through the nuclear force, and neutrons are required for the stability of nuclei. Neutrons are produced copiously in nuclear fission and fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes.
The neutron is essential to the production of nuclear power. In the decade after the neutron was discovered in 1932, neutrons were used to induce many different types of nuclear transmutations. With the discovery of nuclear fission in 1938, it was quickly realized that, if a fission event produced neutrons, each of these neutrons might cause further fission events, etc., in a cascade known as a nuclear chain reaction. These events and findings led to the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity, 1945).
Free neutrons, or individual neutrons free of the nucleus, are effectively a form of ionizing radiation, and as such, are a biological hazard, depending upon dose. A small natural "neutron background" flux of free neutrons exists on Earth, caused by cosmic ray showers, and by the natural radioactivity of spontaneously fissionable elements in the Earth's crust. Dedicated neutron sources like neutron generators, research reactors and spallation sources produce free neutrons for use in irradiation and in neutron scattering experiments. Wikipedia, Neutron
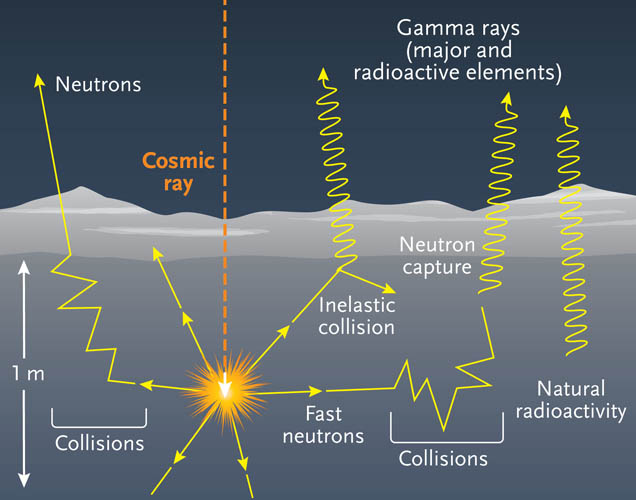
Discovery
In 1930 Walther Bothe and H. Becker in Germany found that if the very energetic natural alpha particles from polonium fell on certain of the light elements, specifically beryllium, boron, or lithium, an unusually penetrating radiation was produced. At first this radiation was thought to be gamma radiation although it was more penetrating than any gamma rays known, and the details of experimental results were very difficult to interpret on this basis. The next important contribution was reported in 1932 by Irene Joliot-Curie and F. Joliot in Paris. They showed that if this unknown radiation fell on paraffin or any other hydrogen-containing compound it ejected protons of very high energy. This was not in itself inconsistent with the assumed gamma ray nature of the new radiation, but detailed quantitative analysis of the data became increasingly difficult to reconcile with such an hypothesis. Finally (later in 1932) the physicist James Chadwick in England performed a series of experiments showing that the gamma ray hypothesis was untenable. He suggested that in fact the new radiation consisted of uncharged particles of approximately the mass of the proton, and he performed a series of experiments verifying his suggestion. Such uncharged particles were eventually called neutrons, apparently from the Latin root for neutral and the Greek ending.
A neutron contains two "down" quarks and one "up" quark, while a proton contains two up quarks and one down quark. Since an up quark (u) has a charge of 2e/3, where -e is the charge of the electron, and a down quark (d) has a charge of -e/3, neutrons are neutral and protons have a positive charge.
Since an up quark (u) has a charge of 2e/3, where -e is the charge of the electron, and a down quark (d) has a charge of -e/3, neutrons are neutral and protons have a positive charge.
Quarks, leptons and baryons are all fermions.
Neutron PHYS An elementary particle which has approximately the same mass as the proton but lacks electric charge and is a constituent of all nuclei having mass number greater than 1.
The proton (and neutron), i.e., nucleur matter, on the other hand, has been shown to be composed of more elementary units, the quarks. Supposedly six different kinds of quarks exist, again grouped in three families, in complete symmetry to the leptons. Five of these quarks have been found, and recent experimental evidence exists for the sixth quark. Only the two members of the first family are needed for "ordinary" nuclear matter, the "u" and the "d" quark. However, the quarks seem to always be bound together to form the nuclear particles, called hadrons, and never have been observed as free particles: Three such quarks make up the proton, two u quarks and one d quark, while the neutron consists of one u quark and two d quarks.
Hadrons containing the heavier quarks, s, c, and b have also been observed. These particles, however, have very short lifetimes and are being produced under "natural" condition[s] mostly in reactions of cosmic rays with nuclei in the terrestrial atmosphere.
The radioactive beta decay is possibly due to the weak interaction, which transforms a neutron into: a proton, an electron, and an electron antineutrino. Wikipedia, Weak Interaction
See Also
Electron
Figure 7B.09 - Feynmans Triplet Structure of Photon
Neutrino
neutrino oscillation
Particles and Corpuscles
Proton
Quark
Sympsionics
Table of Quantum Particles