Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, ?. The symbol for the alpha particle is ? or ?2+. Because they are identical to helium nuclei, they are also sometimes written as He2+ or 4 2He2+ indicating a helium ion with a +2 charge (missing its two electrons). If the ion gains electrons from its environment, the alpha particle can be written as a normal (electrically neutral) helium atom 4 2He. Wikipedia, Alpha Particles
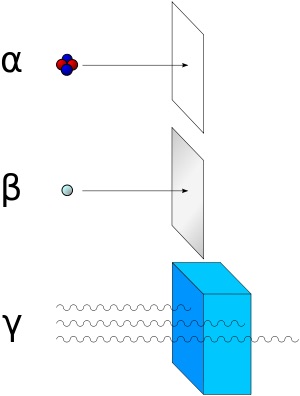
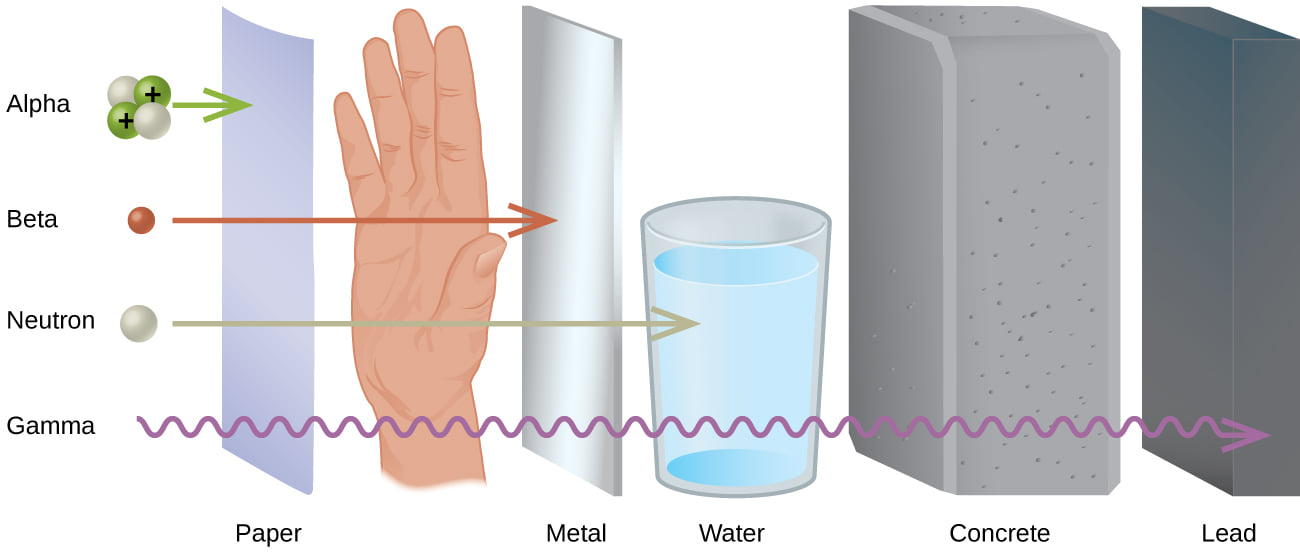
Alpha radiation consists of helium nuclei and is readily stopped by a sheet of paper. Beta radiation, consisting of electrons or positrons, is halted by an aluminum plate. Gamma radiation is dampened by lead.
Russell
"The reverse of this principle applies in depolarizing bodies. Depolarizing bodies on the radioactive half of any cycle project time accumulations from them at tremendous speeds. Helium and other inert gases explode outwardly from tungsten at approximately half the "speed of light" while similar "rays" explode outwardly from radium, actinium, thorium, uranium and uridium at almost the speed of light.
Conversely, generoactive rays explode inwardly at tremendous speeds in the first three invisible octaves. Alpha, beta, gamma and "cosmic" rays explode inwardly to center invisible generating matter as they and the older inert gases explode outwardly from degenerating visible matter." [Walter Russell, A New Concept of the Universe, pages 112-117]
Element 115 decays and produces alpha particles. These alpha particles can amplify plasma wave fluctuations, with the specific mechanism depending on the characteristics of the plasma and the kinetic effects of the alpha particles. The main reasons include:
1. Resonant Instability
Alpha particles can resonate with plasma waves, injecting energy into them and thereby enhancing wave intensity. For example, in magnetized plasma, alpha particles may undergo Landau resonance or cyclotron resonance with Alfvén waves or ion-acoustic waves, leading to wave instability growth.
2. Driving Turbulence
In nuclear fusion devices (such as tokamaks) or astrophysical environments (such as the solar wind), alpha particles are high-energy ions. Their injection and dissipation can drive plasma turbulence, which amplifies small-scale fluctuations and facilitates energy transfer across different scales.
3. Free Energy Injection
Alpha particles are typically much hotter than the background plasma, providing additional free energy that triggers wave growth. For example, in magnetically confined fusion, the energy injected by alpha particles can excite waves (such as TAE—Toroidal Alfvén Eigenmodes) in tokamak plasmas, leading to instability growth.
4. Nonlocal Effects
Alpha particles can induce anisotropic plasma distributions, altering wave growth rates. For instance, in space plasma environments like the solar wind, the drift velocity of alpha particles differs from that of protons. This velocity difference can influence the intensity of waves such as Alfvén waves.
Thus, alpha particles can indeed amplify plasma wave intensity, primarily through mechanisms like resonance, instability driving, and free energy injection. The specific impact depends on the energy and density of the alpha particles as well as the background plasma conditions.
This is also why Element 115 is added to the plasma reactor in UFO propulsion systems.
Bob Lazar did not lie!
See Also
Figure 3.00 - Infinite Number of Atomoles or Alphanon filling all Space
Figure 9.14 - Wave Flow and Phase as function of Particle Rotation
Figure 9.15 - Wave Flow and Wave Length as function of Particle Oscillatory Rotation
gamma rays
Particles and Corpuscles
Radiation
Table of Quantum Particles
Uranium
