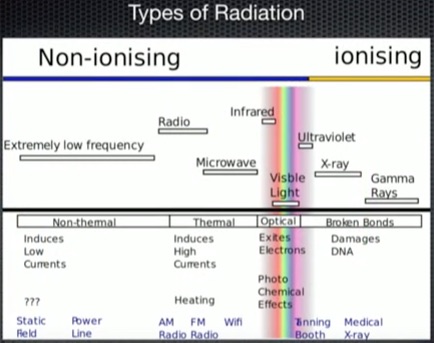
Radiation (click to enlarge)
| Photoelectric Effect (eV)
WR# Indig Element Work Function 502+
2 Berylium
5.00 Photoelectric Effect: It's been determined experimentally that when light shines on a metal surface, the surface emits electrons. For example, you can start a current in a circuit just by shining a light on a metal plate. A freshly polished, negatively charged zinc plate looses its charge if it is exposed to ultraviolet light. This phenomenon is called the photoelectric effect. Careful investigations toward the end of the nineteenth century proved that the photoelectric effect occurs with other materials, too, but only if the wavelength is short enough. The photoelectric effect is observed below some threshold wavelength which is specific to the material. Especially the fact that light of large wavelengths has no effect at all even if it is extremely intensive, appeared mysterious for the scientists. Albert Einstein gave an explanation in 1905: Light consists of particles (photons), and the energy of such a particle is proportional to the frequency of the light. There is a certain minimum amount of energy (dependent on the material) which is necessary to remove an electron from the surface of a zinc plate or another solid body (work function). If the energy of a photon is bigger than this value, the electron can be emitted. Note - Einstein was only partially correct. His description of the observed phenomenon was correct but his causes were contrived Newtonian ideas and not causitive at all. Notice how similar this effect is to Russell's Principle of Regeneration as also his description of how magnetic flow creates/generates an electric flow. |
See Also
See Also
Atomic Cluster Ionization Electromagnetic Radiation ELECTROMAGNETIC RADIATION - Snell Entropy ionization Ionization Energy Penning Ionization Photoionization Radiation Valence
